Computer Simulations First Step Toward Designing New, More Efficient Amine Chemical Scrubbers
A proof-of-concept molecular modeling study from North Carolina State University analyzes the efficiency of amine solutions in capturing carbon dioxide. This series of new computer models is the first step toward the design of cheaper, more efficient amine chemicals for capturing carbon dioxide – and reducing harmful CO2 emissions – in industrial installations.
Industrial scrubbers use chemical solutions to capture carbon dioxide (CO2) from fuel and combustion gas. The scrubbers are a commonly used method for decreasing carbon emissions from industries such as coal-fired power plants, which produce more than 14 billion metric tons of carbon each year. However, amine scrubbing is a costly process, so researchers are constantly looking for new amine chemicals with more desirable qualities such as fast absorption rates, high CO2 capacity, and low heat of reaction.
Denis Fourches, assistant professor of chemistry at NC State, and postdoctoral researcher Melaine Kuenemann wanted to find out if they could create computer models that could predict an amine’s absorption properties based on its chemical structure.
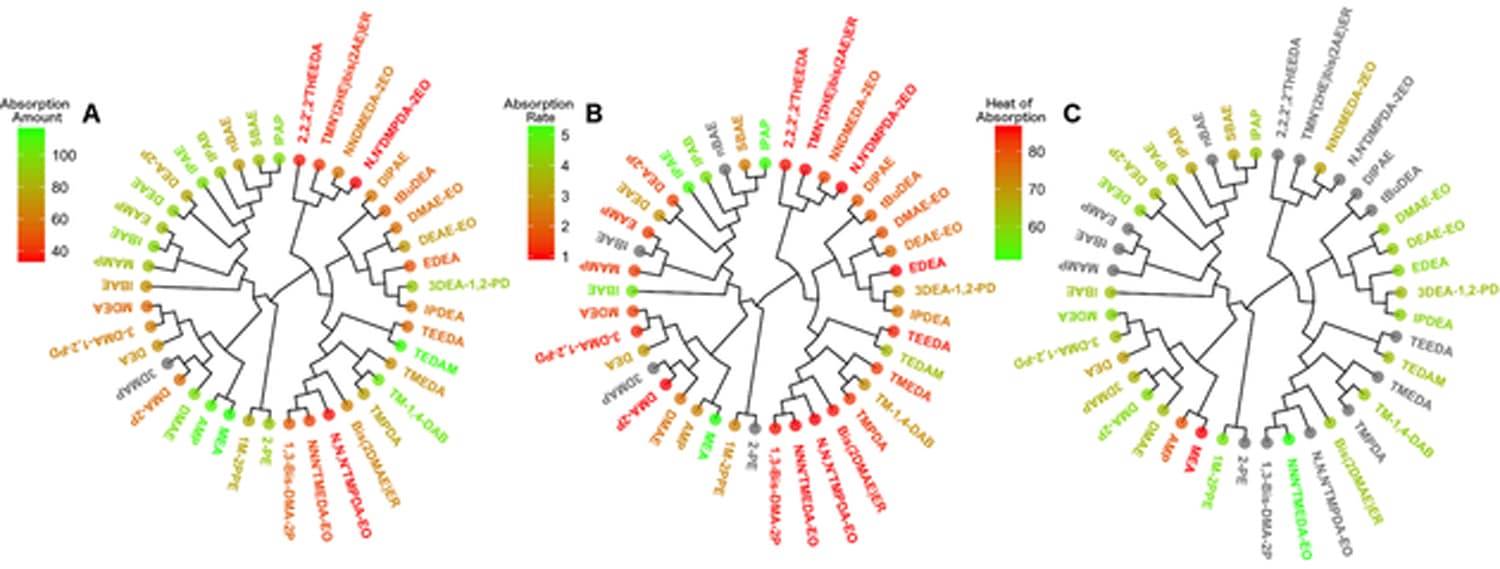
First, the researchers compiled information on 41 publicly available amine solutions and all their chemical and absorption properties. They analyzed the chemical and structural characteristics of each amine, and grouped them into families of chemicals with similar structural properties. Then they looked at how well and how quickly these amines could absorb carbon. Using this data, they created a series of models – known as a quantitative structure-property relationships, or QSPR models – that can predict the amines’ CO2 absorption properties solely based on amines’ structural characteristics.
These models utilize machine-learning techniques – the same ones used by companies like Netflix or Amazon that “learn” a customer’s preferences and make recommendations based upon that data – in order to predict which chemical structures are likely to have the best overall CO2 absorption properties. The models were found to be capable of reliably discriminating between amines with high absorption properties versus those that were less efficient.
“This work is the first attempt to develop computer models for fully evaluating and predicting carbon dioxide absorption properties of amine solutions,” Fourches says. “The next step for us is to utilize these computer models to screen a virtual library of hundreds of thousands of new amines, and identify some new amine candidates predicted to have way better carbon absorption properties.
“If you had to test all of these thousands of compounds experimentally, it would take decades of work,” Fourches continues. “With the powerful computers we have access to, this virtual screening can be done in a matter of days and is very inexpensive. This is a game changer for designing and prioritizing new compounds.”
The research appears in Molecular Informatics. The work was funded by the NC State Chancellor’s Faculty Excellence Program.
-peake-
Note to editors: An abstract of the paper follows.
“Cheminformatics Modeling of Amine Solutions for Assessing their CO2 Absorption Properties”
Authors: Denis Fourches, Melaine Kuenemann, North Carolina State University
Published: March 7, 2017, in Molecular Informatics
DOI: 10.1002/minf.201600143
Abstract: As stricter regulations on CO2 emissions are adopted worldwide, identifying efficient chemical processes to capture and recycle CO2 is of critical importance for industry. The most common process known as amine scrubbing suffers from the lack of available amine solutions capable of capturing CO2 efficiently. Tertiary amines characterized by low heats of reaction are considered good candidates but their absorption properties can significantly differ from one analogue to another despite high structural similarity. Herein, after collecting and curating experimental data from the literature, we have built a modeling set of 41 amine structures with their absorption properties. Then we analyzed their chemical composition using molecular descriptors and non-supervised clustering. Furthermore, we developed a series of quantitative structure-property relationships (QSPR) to assess amines’ CO2 absorption properties from their structural characteristics. These models afforded reasonable prediction performances (e. g., Q2 LOO= 0.63 for CO2 absorption amount) even though they are solely based on 2D chemical descriptors and individual machine learning techniques (random forest and neural network). Overall, we believe the chemical analysis and the series of QSPR models presented in this proof-of-concept study represent new knowledge and innovative tools that could be very useful for screening and prioritizing hypothetical amines to be synthesized and tested experimentally for their CO2 absorption properties.
This post was originally published in NC State News.
- Categories:


