The Science of a Startup

In an underwater world filled with life forms hanging all over one another, a Caribbean sea sponge stands alone.
Around it, colorful reefs and rocks are covered with rough-looking piles of bacteria and other organisms, a phenomenon known as biofouling. But the brown sea sponge, agelas conifera, remains smooth and uncluttered.
Why?
The answer can reshape how doctors treat people with life-threatening diseases such as cystic fibrosis and the deadly staph infection known as MRSA. At the forefront of the work is Dr. Christian Melander, the Howard J. Schaeffer Distinguished Professor of Chemistry at NC State, who has harnessed the sponge’s fouling-fighting power against stubborn bacteria that resist life-saving antibiotics.
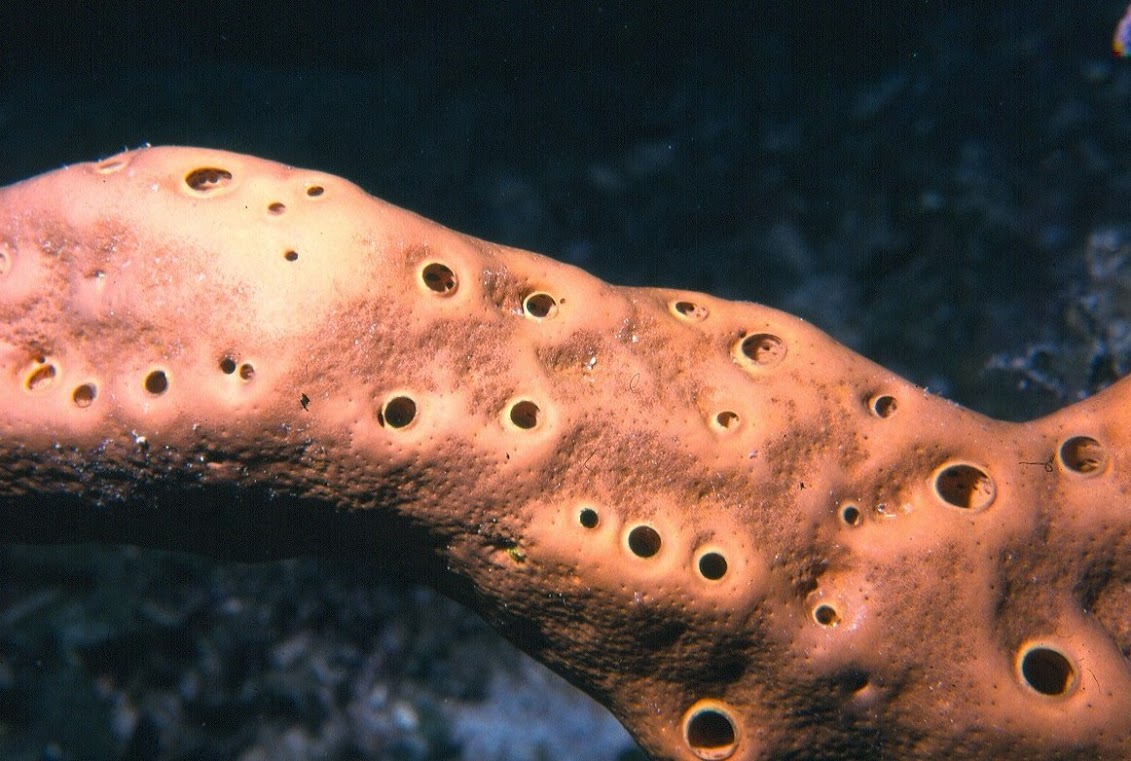
Credit: Sven Zea, The Sponge Guide
Working with Dr. John Cavanagh, the William Neal Reynolds Distinguished Professor of Molecular and Structural Biochemistry, Melander co-founded a company called Agile Sciences that is finding new ways to help antibiotics do their jobs. In 2014, the company landed two new patents and a $1.5 million grant from the National Institutes of Health (NIH) to further its work.
“Part of the benefit of this job is the ability to engage in economic development and the transition of research for the betterment of society,” Melander said. “When you have something that looks promising, I think it should be expected that you make that transition.”
The work began about a decade ago when the researchers noticed that the brown sea sponge — which is immobile and has no immune system — stayed clean while the rest of the ocean floor was covered with fouled surfaces.
That fouling was caused by clustering bacteria that had encased themselves in protective barriers called biofilms. The sticky, pale-yellow gunk a dental hygienist scrapes off teeth is a common form of biofilm, but other types destroy crops, foul ship hulls and coat medical devices. Biofilms are also responsible for many of the 23,000 deaths in the U.S. each year from antibiotic-resistant infections.
The sponge, however, had a secret weapon: a molecular compound that kept biofilms from forming. Melander, armed with an understanding of the sponge’s chemical processes, created chemical compounds that mimic those found in the sponge.
The biomimickry approach worked, and today the company creates all sorts of compounds that fight biofilms.
“Our strategy is to make a molecule that prevents bacteria from forming into a biofilm — or, if the bacteria are already in a biofilm state, create a molecule that will take them out of it,” Melander said.
The work is particularly valuable to people suffering from cystic fibrosis. These patients accumulate thick, sticky mucus in their lungs that provide a wet, warm and comfortable environment for biofilm-protected bacteria to grow. The result is a life-threatening chest infection that provokes a powerful immune response, but the body ends up doing more damage to healthy lung tissue than the bacteria.
“The average lifespan for someone with cystic fibrosis is around 37 years—and that’s with aggressive microbial treatment,” Melander said. “If you can break up the biofilm in CF patients and get rid of their bacteria, you can save the body from itself and maybe extend their lives by decades.”
In 2014, Agile Sciences received a $1.5 million NIH small business grant to develop cystic fibrosis therapies based on the company’s anti-biofilm agents. Agile plans to test the compound on animal lungs and see how well it clears infection. If successful, human trials would follow.

Though fighting biofilms remains one of Agile Sciences’ biggest objectives, Melander says the company and his lab have branched out and now explore a wide range of antibiotic resistance mechanisms.
MRSA is among their targets.
Every year, tens of thousands of Americans check into hospitals hoping for a speedy recovery, only to develop a potentially deadly infection. Methicillin-resistant staphylococcus aureus (MRSA) is an organism that has made its way into virtually every hospital in the United States. Though the bacteria are generally harmless in the general population (most people carry staph at some point in their lives), they become a serious problem when the bacteria enter the body through surgical wounds.
MRSA either creates a biofilm or makes genetic changes that prevent an antibiotic from disrupting its cell structure. Melander’s company is exploring genetic mechanisms in bacteria that, when deactivated, can decrease their resistance capabilities.
One of Agile Sciences’ compounds renders the bacteria unable to recognize the antibiotic as a threat, essentially stopping the defensive process before it can begin.
“Antibiotic resistance is a serious health problem,” Melander said. “But by moving our research from the lab to the marketplace, we’re hoping to create new treatments that can save lives.”
- Categories:


